The Ethical Review Board of the Midwives College of Utah exists to protect the interests of the human subjects involved in student, staff, faculty, and affiliate research. It is required for all research projects conducted through the College. ERBs are also commonly called Institutional Review Boards (IRBs) or ethics review committees; these terms are synonymous in nature. There are four primary phases:
- Initial review and approval process for new research projects
- Ongoing supervision of current research projects
- Review and renewal (if applicable) of research projects, annually
- Final report and closing of studies
We’ll be going over each of these phases individually. Please visit “Forms Central” for all relevant forms with more detailed instructions.
Initial Approval Process
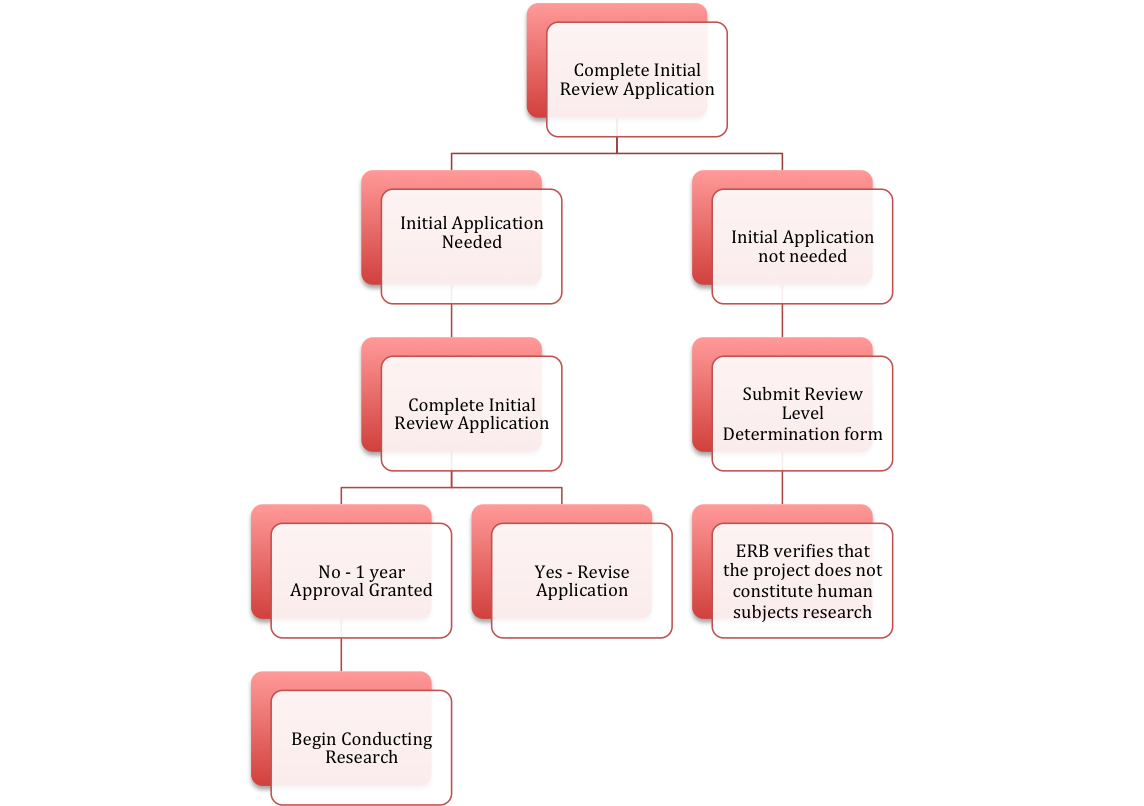
All new human subjects research projects must go through the ERB approval process. There are 3 levels of review that a project involving human subjects may go through. Researchers will begin by filling out the Review Level Determination Form as a self-evaluation. This form is intended to assist researchers in determining whether or not a project involves research with human subjects and if so, the level of ERB review required:
Full review – for those studies that involve human subjects and a) involve some degree of risk, and/or b) involve research with a recognized vulnerable population. An example might be a trial of an herbal treatment or labor management technique.
Expedited review – for those studies that involve human subjects, but only involve minimal risk. An example of this might be a survey asking women what factors they considered in choosing a birth place or attendant.
Exempt review – for those projects that only indirectly involve human subjects. An example might be a study using the MANA Stats database, a vital records database, or another already existing limited dataset.
If the project does not constitute human subjects research, then the researchers should complete and submit the Review Level Determination Form. If the project does constitute human subjects research, the researchers should complete and submit the Initial Application (note: if you are submitting an Initial Application, you do not need to also submit a Review Level Determination Form). Please keep in mind that the decision about whether a project constitutes human subjects research, and which level of review is required, is ultimately a decision of the ERB. A reviewer may be asked to revise their protocol to insure ethics compliance and to meet ERB regulations at any point in the review and approval process.
When the initial approval is granted, it is valid for a period of one year from the date of approval. No research may be conducted, or data gathered, until after initial approval is granted.
The flow chart below will show you how the process of initial approval works:
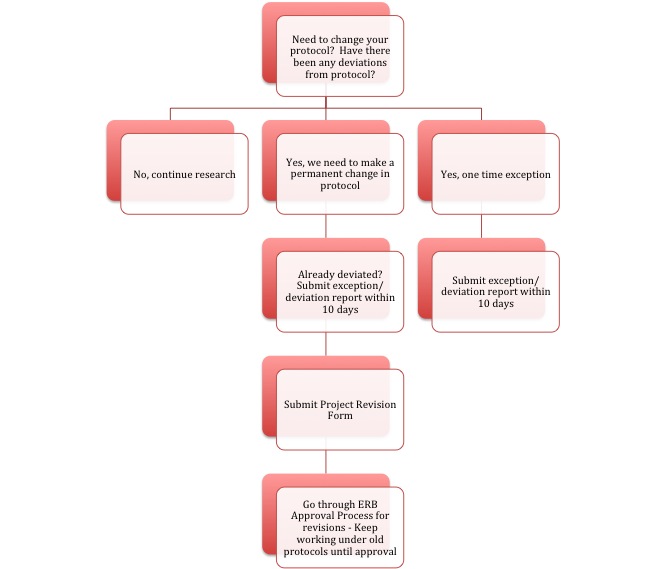
The ERB makes sure that current research is staying within the bounds of ethics by requiring researchers to report any changes or deviations from approved protocol.
If at any time circumstances require the researcher to temporarily deviate from the approved protocol, or make an exception, they must submit an Exception/Deviation Report to the ERB within 10 days of the exception/deviation occurrence. The ERB will evaluate the circumstances and respond to the researcher with any requirements or feedback they deem necessary.
Should the researcher decide to make a permanent change to the protocols of the research, they should submit a Project Revision Form to the ERB before operating under the new protocol. Once the revision has been approved, research may continue under the new protocol.
The flowchart below illustrates these ongoing supervision processes. Investigators should frequently think about ERB requirements and timelines; it is the responsibility of the investigators to insure deadlines and associated requirements are met during the period of ERB approval and active research. (click to view full size)
Review of Research Projects, Annually
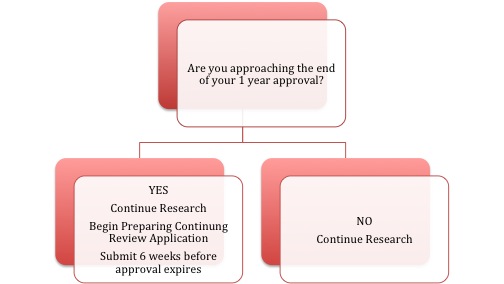
Each ERB approval is good for one year. At the end of the one year approval, the researchers must submit the Continuing Review Application to the ERB to gain approval for another year.
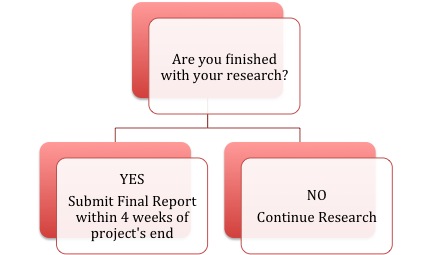
When the research project is finished (meaning all data collection and analysis activities are complete), the researchers must submit the Final Report to the ERB within 4 weeks of the time they finish conducting research. The ERB will review these reports and close out the file on the study at that time. The flowchart below illustrates these steps: (click to view full size)
For more detail on each of these steps, please read The MCU ERB Policy.